No one anticipated how quickly Omicron would sweep the globe. Although the surge from the variant is starting to decline in many countries, worldwide case numbers are still on the rise. The last full week of January saw about 23 million confirmed new cases; previous peaks topped out at about 5 million per week. Beleaguered public-health officials are still scrambling to curtail the virus’s spread so that people with COVID-19 don’t overwhelm the hospitals.
Omicron also presented immunologists with a new and urgent puzzle. Initial data suggest that existing vaccines, designed around the original SARS-CoV-2, do not provide much protection from becoming infected with the variant, even if they do seem to reduce the risk of hospitalization or death. The protection provided by two doses of a messenger RNA vaccine drops to less than 40% just a few months after the second dose1,2. But a third, ‘booster’ dose seems to help. One report found about 60–70% protection from infection at two weeks after a third shot1, and protection from severe illness seems strong2.
“This is very exciting,” says Mark Slifka, an immunologist at Oregon Health & Science University in Portland. It’s also a little surprising. Why would a third encounter with a vaccine targeted to the original virus’s spike protein — which it uses to enter cells — work against this variant, which has more than 30 mutations in the spike?
The human immune system’s ability to remember past infections is one of its hallmarks, but a durable response is not guaranteed. Some infections and immunizations elicit lifelong protection, but for others, the response is modest and requires regular reminders in the form of booster shots or new, reformulated vaccines. COVID-19 has forced on the world a chance to explore the intricacies of this complex and crucial biological phenomenon. “It’s an amazing natural experiment,” says Donna Farber, an immunologist at Columbia University in New York City. “It’s just this unbelievable opportunity to look at human immune responses in real time.”
With around ten billion shots of a dozen COVID-19 vaccines already in people’s arms, and five worrying variants pulsing around the globe, scientists are scrambling to answer key questions. How long will vaccination protect people for? What will that protection look like? And, of course, how will a vaccine developed against the original SARS-CoV-2 fare against other variants, such as Omicron?
“We are just at the beginning of a wave of discovery,” says John Wherry, an immunologist at the University of Pennsylvania’s Perelman School of Medicine in Philadelphia. What emerges will be crucial not only for fighting COVID-19, but for understanding some of the most fundamental features of immune memory.
Making memories last
The immune system kicks into action soon after a pathogen enters the body. But it can take several days for the specialized cells that target viruses and bacteria to join the battle. These B cells and T cells work to eradicate the infection; after the fight is over, they remember the intruder.
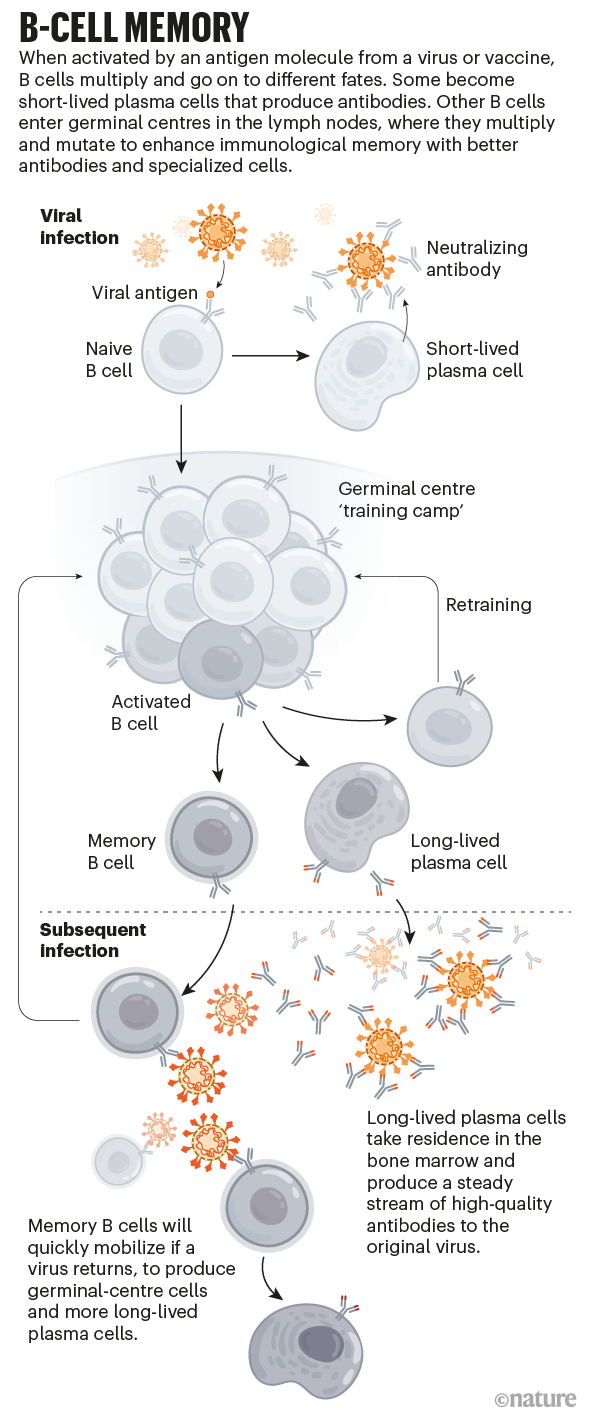
B cells “are the first responders”, says Ali Ellebedy, an immunologist at the Washington University School of Medicine in St. Louis, Missouri. During a first exposure to a pathogen, B cells that get activated divide rapidly and differentiate into plasma cells that churn out proteins called antibodies. Antibodies can flag suspicious intruders for destruction, and some might bind to a part of a pathogen that prevents it from infecting cells altogether. These are the ‘neutralizing’ antibodies. “They’re the only thing that can truly give you sterilizing immunity,” says Shane Crotty, an immunologist at the La Jolla Institute for Immunology in California. That’s why researchers typically use the presence of these antibodies as a proxy for immune protection.
By September 2020, a handful of studies3,4 reported that neutralizing-antibody levels were dropping in people who had recovered from COVID-19. Some experts expressed alarm that immunity to SARS-CoV-2 might therefore be fleeting.
Immunologists, however, weren’t surprised. Antibodies are supposed to wane after an infection. The short-lived B cells that churn out antibodies right away die off quickly. “This is something we’ve known forever,” says Rafi Ahmed, an immunologist and director of the Emory Vaccine Center at Emory University in Atlanta, Georgia.
What matters is whether the body makes long-lived B cells that can target the pathogen if it reappears. These cells typically develop inside structures called germinal centres, which arise in the lymph nodes during an infection and serve as a sort of B-cell training camp. There, the cells multiply and acquire mutations. Only those that produce the best antibodies, the ones that latch most securely on to the surface of the virus, survive. It’s “almost a winnowing process”, Ellebedy says.
Within a month or so, some of the cells that produce these super-binders become memory B cells that circulate in the blood (see ‘B-cell memory’). They don’t produce antibodies, but if they encounter the virus or its proteins, they can rapidly divide and become plasma cells that do. The rest become long-lived plasma cells that reside mainly in the bone marrow and secrete a small-but-steady stream of high-quality antibodies. “Those cells basically live with us for the rest of our lives,” Ellebedy says.
A drop in antibody levels after infection is normal. What immunologists really want to know is where — or whether — the decline will stop. In April 2020, Ahmed and his team began studying people who had recovered from COVID-19. The scientists found that those people’s antibody levels dropped quickly for the first two or three months after infection. But then, after about four months, the researchers saw the curve start to flatten. They have published results on the first eight months5, but now have data up to 450 days, and Ahmed is encouraged by what they see. So far, “looking at the shape of the curve, it looks pretty damn good”, he says. “It is really quite stable.”
The immune response after vaccination more or less mimics what happens after infection, with one major difference. In a SARS-CoV-2 infection, the immune system sees the whole virus. The most effective vaccines, however, are using just one viral protein to elicit a response: spike. And whether antibody levels will also plateau after vaccination isn’t yet clear. Wherry and his colleagues analysed immune responses in 61 people for 6 months after their first shot, finding that antibody levels peaked about a week after the second shot and then fell quickly for a couple of months. After that, they declined more slowly6.
With that decline came a drop in protection. The shots, which became widely available in some countries as early as December 2020, showed impressive effectiveness initially. But by July 2021, reports began to surface of breakthrough infections. Data from Israel, which had launched an aggressive vaccination campaign using the Pfizer–BioNTech mRNA vaccine, suggested that this vaccine’s protection against infection dropped from 95% to just 39% over the course of 5 months (see go.nature.com/3hjdxtn; in Hebrew and English).
Those numbers make it sound as though the vaccine is faltering. And researchers have seen that, over time, it does lose its ability to keep infection at bay. But vaccines have retained their ability to prevent serious illness. Protection from infection might be waning, but protection against hospitalization seems to be holding up. “You’re probably going to have protective immunity for years,” Crotty says.
The cells will save us
Immune memory depends on more than just antibodies. Even when antibody levels drop, memory B cells can recognize a return invader, divide, and quickly start churning out antibodies to fight it. And the memory B-cell response improves over time, at least in the short term. Six months after vaccination, the individuals in Wherry’s study6 had elevated numbers of memory B cells that responded not only to the original SARS-CoV-2, but also to three other variants of concern.
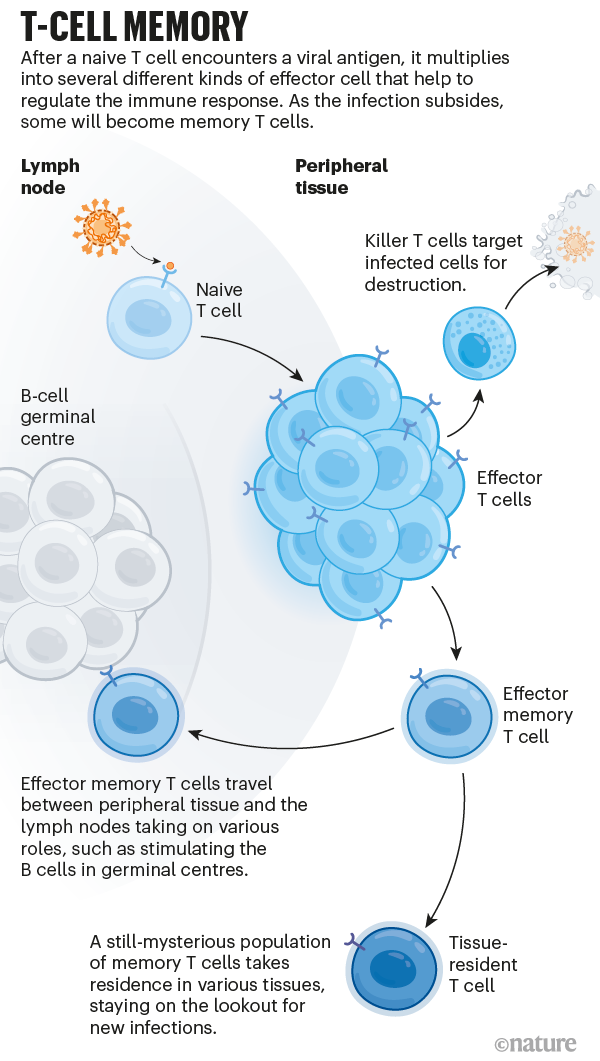
And then there are T cells, the third pillar of immune memory. On coming into contact with an antigen, these multiply into a pool of effector cells that act to wipe out the infection. Killer T cells quickly divide to assassinate infected cells, and various types of helper T cell secrete chemical signals that stimulate other parts of the immune system, including B cells. After the threat has passed, some of these cells persist as memory T cells (see ‘T-cell memory’).
Some people might carry memory T cells from past coronavirus infections — such as those that cause common colds — that can recognize SARS-CoV-2. These cells could help to fight the infection, or even stop it completely. One study7 found that health-care workers who were exposed to SARS-CoV-2 but never tested positive had subtle signs of a response to infection. The researchers hypothesize that cross-reactive T cells shut the infection down before it could take hold. “These people did have an infection in a sort of loosest sense of the word,” says Mala Maini, an immunologist at University College London who led the study. But “there’s probably not much virus around because it’s being shut down very quickly”.
This idea is still controversial, and the phenomenon might be rare. Memory cells typically can’t block infection in the way that neutralizing antibodies can, but they don’t necessarily need to. With COVID-19, infection happens quickly, but it takes a little while to cause serious illness. That gives memory T cells some time to do their jobs. When re-exposed to a virus or booster, these cells will kick into overdrive, “proliferating like crazy”, Crotty says. “In a 24-hour period, you can get a tenfold increase in the number of your memory T cells.” That’s probably not fast enough to have much of an effect on getting sick, he adds. But it could be fast enough to prevent hospitalization.
And it’s much harder for the virus to find a way around the T-cell response. That’s because T cells in one individual recognize different parts of the virus than do T cells in another individual. So a virus could mutate to escape one person’s T-cell response, but not another’s. “Escape is meaningless at the population level,” Crotty says. Also, T cells can see parts of the virus (or the spike protein) that antibodies can’t, including pieces that are less likely to mutate.
Several studies have found that people who had been vaccinated or had been infected with SARS-CoV-2 had about the same T-cell response to Omicron as they did to the Delta variant, despite the large number of mutations8,9. Observations of Omicron’s spread also suggest that this is so. A T-cell response is possibly also helping to drive the phenomenon known as ‘decoupling’. In areas with higher immunity because of past infections or vaccination, the number of cases of Omicron has risen quickly, but the number of hospitalizations and deaths has increased much more slowly.
Evolution of immunity
A perfect vaccine would induce an immune response that is not only durable, but also broad enough to protect against the virus as it mutates and evolves. With Omicron raging, it seems the vaccines have lost some ground. But the immune system still has a number of tricks to deal with viruses that keep changing.
One of those tricks happens inside the germinal centres. There, the B-cell training not only improves how well antibodies bind to their original target; it can also boost the number of binding sites they recognize, increasing the odds that they can identify a variant.
“Indirectly, the whole success of vaccination depends on how robust the germinal centre is,” Ellebedy says. Dogma suggests that without the germinal centre, “we don’t have memory”.
But that might not be entirely true. The immune system has “a grab bag of other pathways” that are more nuanced and less well studied, says Stephanie Eisenbarth, director of the Center for Human Immunobiology at Northwestern University Feinberg School of Medicine in Chicago, Illinois. Research by Eisenbarth and her colleagues shows that even mice that lack the ability to make germinal centres can generate long-lived plasma cells10. How these cells arise isn’t entirely clear, but just like the plasma cells that come through the germinal centre, these seem to bind tightly to their targets.
Emerging data suggest that Omicron is, nevertheless, able to largely circumvent the antibodies generated by past infection or vaccination. Pfizer reported a 25-fold drop in the neutralization of Omicron (compared with the original SARS-CoV-2) in people who had received two vaccine doses. Why a third-dose booster might bring back protection isn’t entirely clear.
It’s possible that a third shot simply boosts all antibody levels equally, including the small proportion that can recognize pieces of Omicron’s spike protein that haven’t changed. “We know already from some of the data released by the companies that antibodies get boosted very, very efficiently,” says Wherry. But it’s looking likely that a third shot actually increases the breadth of the response.
In one study11, researchers assessed blood from people who had received vaccines from Moderna, Pfizer–BioNTech or Johnson & Johnson to assess how well their antibodies neutralized a virus containing spike protein from SARS-CoV-2 variants. Blood from individuals who received one or two doses had little ability to neutralize Omicron. But blood from people who had received a booster dose of an mRNA vaccine fought the variant effectively. Their neutralization capacity against Omicron was only four- to sixfold lower than against the original strain.
People who have received two doses of vaccine have memory B cells that can bind to Omicron12. It’s possible that a third shot prompts these memory cells to become antibody-producing cells. “One of the major jobs of memory B cells is to be a library of guesses by the immune system about what a variant may look like,” Crotty says.
Wherry offers another possibility. The booster might be triggering the formation of germinal centres, setting off another cascade of mutation among B cells. “That’s one of the things that we’re going to be watching carefully,” he says.
Slifka posits that the first dose of the vaccine generates antibodies that bind well to the features of the spike protein that are readily accessible. When subsequent doses arrive, existing antibodies quickly coat those accessible features, leaving less-accessible targets available for B cells to latch on to.
The good news about boosters, however, comes with a caveat. It’s not clear how long booster protection will last. Data from the United Kingdom suggest it could wane quickly13. Three doses of the Pfizer–BioNTech vaccine provided 70% protection initially. But by 10 weeks, protection against infection had dropped to 45%. And reports emerging from Israel suggest that a fourth-dose booster doesn’t seem to elevate protection effectively. This suggests that the best next move might be to develop Omicron-specific booster shots.
Pfizer and Moderna are already working on mRNA versions of such jabs. In January, Pfizer chief executive Albert Bourla said that an Omicron-specific vaccine should be ready to launch by March. By then, however, many will already have been infected with the variant and gained some immunity that way. Pfizer is also working on a shot that would include both the original spike and one from Omicron. The ultimate goal, of course, is to develop a jab that would provide long-lasting immunity without multiple boosters.
The magic ingredient
SARS-CoV-2 could provide other opportunities for learning how to improve vaccination. In 2019, Slifka and his colleague Ian Amanna published a review14 looking at different types of vaccine and hunting for patterns that might help to predict why some induce durable immunity and others don’t.
Of the vaccine types they looked at, the longest-lasting protection tended to come from live-virus vaccines. These consist of pathogens that have been altered so that they can’t cause disease. Because they mimic the actual infection so well, they tend to elicit a durable response. But those that contained whole inactivated virus or pieces of viral protein elicited good memory, too. What seems to matter, Slifka says, is the amount of time the antigen sticks around. “You don’t have to be chronically infected,” he says, “but it has to maintain stimulation of the immune system for a certain amount of time.”
Slifka and Amanna didn’t include mRNA vaccines in the paper — the technology wasn’t in common use — but these do seem to fit the trend. For mRNA vaccines, the antigen gets produced by cells in the body (from an mRNA template). It sticks around for just a few weeks. And the evidence so far suggests that immunity might also be transient. But RNA vaccines that have the ability to replicate in the body might bring about longer-lasting immunity.
SARS-CoV-2 has given scientists a plethora of vaccines to observe and compare against the backdrop of an active pandemic, including those using whole, inactivated virus; protein; or mRNA, or those based on an adenovirus, such as Oxford–AstraZeneca’s or Johnson & Johnson’s offerings. There have been surprises. The response after a shot of the Johnson & Johnson vaccine, for example, elicits a weaker immune response than the mRNA vaccines initially, “and then it actually starts to get better over time”, says Deepta Bhattacharya, an immunologist at the University of Arizona in Tucson. “Something interesting is happening there.”
Scientists are also eager to understand what happens when people mix and match vaccines. A UK study known as Com-CoV has been investigating this phenomenon since early in the pandemic. Its most recent data15 show that people who received a first dose of either Oxford–AstraZeneca or Pfizer–BioNTech followed by Moderna had a higher antibody response than those who received a second dose of the same vaccine.
“You can think about it like cross training,” Wherry says. Mixing and matching different kinds of vaccine might create a more flexible, diverse immune memory.
Adding more targets might also trigger better protection. The most effective current vaccines target the spike protein, but T cells can see the whole virus, says Bali Pulendran, an immunologist at Stanford University in California. He thinks of immunological memory as an enormous chandelier suspended by three thin wires: one represents the antibody response, one is memory B cells and the third is memory T cells. Each is important and should be considered in vaccine design. If one or two of the strands were severed, “would we be confident standing under it?” Pulendran asks.
A shot with broad, durable neutralizing activity against SARS-CoV-2 was always going to be a tall order. Much of that comes down to the nature of the virus itself. “If you look at respiratory infections, these historically have been very hard to prevent,” Ahmed says. That applies to influenza, respiratory syncytial virus and “we definitely see it with the common cold”. With a systemic infection, such as measles, it takes time for the virus to spread through the body and cause illness. With respiratory infections, it’s happening right at the point of entry. For such pathogens, protecting against serious illness might be the best anyone can hope for.
Many are still optimistic, however. “Everyone and their mother is studying SARS-CoV-2 right now,” says Scott Hensley, an immunologist at the Perelman School of Medicine. That surge of interest has led to remarkable advances in immunologists’ ability to dissect the immune response. The insights might finally help them to unlock the recipe for a vaccine that offers long-lasting, broad protection.
“What’s the magic sauce?” Pulendran asks. “Therein lies a deep, deep mystery, a fundamental challenge, which if it is solved will have a transformative effect on vaccinology.”