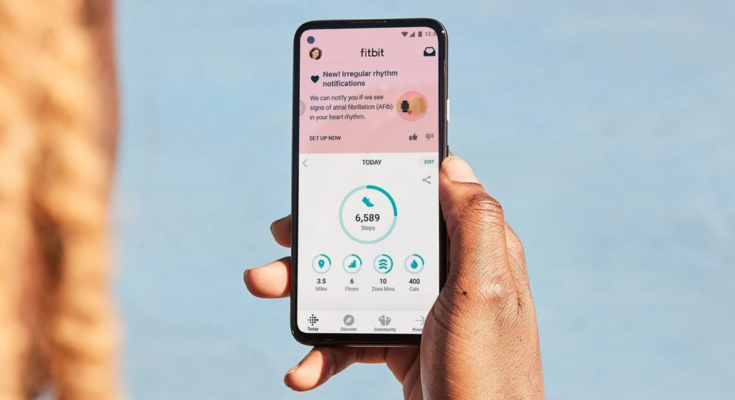
The Food and Drug Administration has given Fitbit the green light to monitor users’ heart rhythms in the background. A new photoplethysmography (PPG) algorithm can passively check a user’s heart rhythm while they’re still or asleep. If the tech detects signs of atrial fibrillation (AFib) — a type of irregular heart rhythm — it will alert the wearer. Fitbit parent Google submitted the algorithm to the FDA for review last month.
Fitbit previously received FDA clearance to use electrocardiogram (ECG) tech in 2020’s Sense Smartwatch. However, that method requires users to run ECG tests manually. Google notes that AFib can be difficult to detect as episodes can be sporadic and pass without any symptoms. Monitoring heart rhythms in the background could improve detection. AFib affects more than 33.5 million people, and those with the condition have a higher risk of stroke.
In May 2020, Fitbit conducted a study of the PPG algorithm which lasted over five months and had more than 450,000 participants. It found that the algorithm correctly identified AFib episodes 98 percent of the time. Google used ECG patch monitors for confirmation.
Fitbit will soon roll out the background heart rate monitoring and Irregular Heart Rhythm Notifications features in the US. They will be available on “a range of heart-rate enabled devices.” Apple Watch 4 and later can also passively monitor heart rhythms for signs of AFib. While neither company’s devices can make a formal diagnosis, they could prompt wearers to consult a doctor for advice should they detect possible AFib.
All products recommended by Engadget are selected by our editorial team, independent of our parent company. Some of our stories include affiliate links. If you buy something through one of these links, we may earn an affiliate commission.